WELCOME

Physiology is the study of function; Human physiology is the study of the function of our bodies. Anatomy is the study of structure; human anatomy is the study of our body structure. Physiology and anatomy go hand and hand, and the study of one subject relates to the study of the other. In this course we will emphasize the study of function, physiology. Much of physiology is how we maintain a constant internal environment. The maintenance of our internal environment is called homeostasis.

|
Cellular Organization
|
To understand physiology, it is best to first understand the way in which the body is organized. An anatomical orientation tells us that our bodies are comprized of many little cells. (Slide 2) A functional perspective looks at the flows of and energy through the systems which comprise the body.
|
Matter
|
|
|
A diagram showing the matter flows in the systems of the body is called a mass flow diagram.(Slide 3) A diagram that shows energy flows in the body is an energy flow diagram.
For the purposes of this course we will be concentration on levels 5 through 8. You should already be familiar with the first four levels, 1 through 4 (we will review these briefly).
Cells - Cells are the smallest self contained functional unit of the body. What we mean by ‘functional' is that, given the proper nutrients and surroundings, environment, a cell can survive; however, if the subcellular organells are removed, they cannot survive.
Tissues - Cells are extremely small, and, individually, they can not do much. Unfortunately, there are inherent transport limitations in just making cells larger and larger so that they can do more. However, it is possible to overcome these limitations. When lots of small cells combine together, they can accomplish as much as a large cell. Therefore, to overcome this limitation, cells similar in structure, function and embryonic origin are grouped together to increase output, these groupings form tissues.
Organs - Since the functions that occur in our bodies are frequently very complex, more than one type of cell/tissue is needed to accomplish a task. These specific grouping of tissues form organs.
Systems - Groups of organs that work together are called systems. The human body is typically divided into 10 organ systems.
|
Fluid Volumes
|
|
|
Our bodies are mostly water. The liquid inside our cells is known as Intracellular fluid. The liquid which surrounds the cells in Interstitial fluid. The Fluid which circulates to supply food, nutrients, and remove wastes, is called Plasma. (Slide 4)

The nerve cells are specialized to initiate and conduct electric signals, often over long distances. A signal may initiate new electric signals in other nerve cells, neuro-neuro junction, it may stimulate secretion by a gland cell, neuro-secretory junction, or cause the contraction of a muscle, neuro-muscular junction. Therefore, nerve cells provide a major means of controlling the activities of other cells. Their activity also underlies such phenomena as consciousness and perception.
Muscle cells are specialized to generate the mechanical forces that produce force and movement. They may be attached to bones and produce movements of the limbs; they may be attached to skin to produce facial expressions, skeletal muscle. They may enclose hollow cavities so that their contraction expels the contents of the cavity as in the pumping of the heart, cardiac muscle, or the emptying of the stomach, smooth muscle. Muscle cells also surround many of the tubes of the body – e.g.; blood vessels.
Connective cells function connecting, anchoring, and supporting the structures of the body. These cells typically have a large amount of extracellular material or matrix between them. Some connective tissue cells are found in the basement layer, the loose meshwork of cells and fibers underlying most epithelial layers; other types include adapose, fat-storing cells, bone cells, tendons, and red and white blood cells. The characteristic of the matrix often determines the characteristic of the tissue type. By convention, the term ‘connective cells' is not used, rather ‘connective tissue' is the way to refer to this type of cell.
Epithelial cells are specialized for protection, secretion and excretion. They are responsible for the selective secretion and absorption of minerals (salts or ions) and organic molecules (carbohydrates, fats, proteins). They are located mainly at the surfaces that either cover the body or individual organs or else line the walls of various tubular and hollow structures such as the intestine or blood vessels.
Organs are composed of the four kinds of tissues arranged in various proportions and patterns.
Organ systems include circulatory, respiratory, digestive, urinary, muscular, skeletal, immune, nervous, endocrine, reproductive, and integumentary.
Respiratory system ![]() takes up oxygen (O2) from the external environment and eliminates carbon dioxide
(CO2) to the external environment
takes up oxygen (O2) from the external environment and eliminates carbon dioxide
(CO2) to the external environment
Circulatory system ![]() movement of blood through the vascular tree; transports O2 from the lungs to
the cells and CO2 from cells to lung; transports nutrients to and wastes from
cells
movement of blood through the vascular tree; transports O2 from the lungs to
the cells and CO2 from cells to lung; transports nutrients to and wastes from
cells
Digestive system ![]() processes nutrients and makes them available to the circulatory system for distribution
to the cells
processes nutrients and makes them available to the circulatory system for distribution
to the cells
Urinary system ![]() regulates the amount of water and minerals in the body and eliminates certain
waste products
regulates the amount of water and minerals in the body and eliminates certain
waste products
Nervous and Hormonal [Endocrine] systems ![]() coordinate and control the activities of the other organ systems
coordinate and control the activities of the other organ systems
Musculoskeletal system ![]() skeletal support and movement
skeletal support and movement
Reproductive system ![]() reproduction
reproduction
Immune system ![]() defense
against foreign invaders and internal malfunctions
defense
against foreign invaders and internal malfunctions
Integumentary system ![]() role of the skin in protection, in temperature regulation, and the housing of
sensory receptors
role of the skin in protection, in temperature regulation, and the housing of
sensory receptors
There are over a hundred of different types of atoms, the different elements, that exist. The most common atoms in biology are:
Mineral elements account for 0.7% of the atoms of the body and include:
A partial list of trace elements is:

|
Methane
|
Two or more atoms bonded together make up a molecule. Methane is comprisedof a carbon atom surrounded by four hydrogen atoms.
A molecule of water (H2O) can be diagramed as O-H, carbon dioxide (CO2) diagramed as O=C=O, and phosphate (PO4) diagramed as a phosphorus atom surrounded by four oxygen atoms.
Atoms are held together with "bonds" to form molecules. In aqueous solutions atoms are held together with loose bonds (ionic).
|
Water Molecules
|
Water Polarity
|
A stronger association-type bonds (hydrogen bonds) are due to electrostatic type attractions. These are found between water molecules due to the polar nature of the water molecule.
|
Covalent bonds
|
Carbon bonds
|
The strongest bond is the covalent bond which is formed by atoms which share electrons. Carbon participates in this type of bonding and is the basis of "organic" chemistry.
a. Definition: particular atoms that, when mixed with water, dissociate from each other and acquire an electric charge
b. examples
common table salt plus water yields sodium ion plus chloride ion
[note: the water molecule notation has been omitted] carbonic acid yields hydrogen ion plus bicarbonate ion
hydrochloric acid yields hydrogen ion plus chloride ion
c. common ions: H+, Na+, K+, Cl-, PO33-, Mg2+, and Ca2+
d. electrolyte vs. non-electrolyte
Because of their ability to conduct electricity, ions are collectively referred to as electrolytes. An example of an important molecule that does not become electrically charged when interacting with water is glucose [one type of sugar]. Therefore, glucose is not an ion; rather it is classified as a non-electrolyte.

A solution is formed when a solid material, such as salt or sugar, "disolves" into a solvent, e.g., a liquid such as water. The solute disassociates into the solvent.
These are solid, liquid, or gaseous substances made up of very small insoluble nondiffusable particles that remain is suspension in a surrounding solid, liquid, or gaseous medium of different matter.
A substance containing two or more ingredients; distinguished from a compound in that the constituents are not in fixed proportions, do not lose their individual characteristics, and can be separated by physical means.

 Table of Organic Molecule Functional Groups
Table of Organic Molecule Functional GroupsBiomolecules - there are four types of biomolecules, these are carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates are molecules composed of carbon, carbo, and water, hydrate. They are of the formula n(CH2O), where n is typically equal to 6.
|
Monosaccharide
|
The simplist unit of the carbohydrate is called the mono (one) saccharide (sugar).
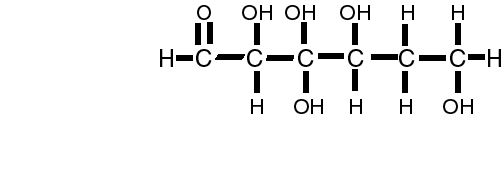
or C6H12O6 or glucose
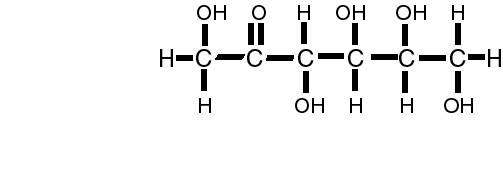
or C6H12O6 or fructose
|
Structural Differences
|
Many of the monosaccharides have the same formula, however, the way that the hydrogen and hydroxyl groups are attached to the carbon are different. A simple change in the orientation/location of the hydroxyl group creates two different sugars!
[It is not necessary to memorize the actual molecular structure of these carbohydrates; however, the basic differences comparing carbohydrates, lipids, and protein are important.]
|
Sucrose
|
Sucrose, or table sugar, is comprised of two monosaccharides, glucose, blood sugar, and fructose, fruit sugar. It is broken down, hydrolized, by an enzyme called sucrase. The "ase" suffix is attached to indicate the enzyme which breaks down the substance, sucrose in this case. These two sugares are important in the process of glucolysis and the production of ATP.
|
Glycogen
|
Glycogen is an animal polysaccharide. It is composed of many glucose monosacharides. It is a useful way of storing many glucose molecules without increasing the tonicity of a solution. The glucose molecules are typicaqlly of an alpha linkage, either 1-4 for a straight chain, or 1-6 for branched chains.
|
Water clusters
|
Water and oil do not mix. This property causes clustering of lipids and the spontaneous formation of structure called micelles. Substances which like water are hydrophylic while those that dislike water are hydrophobic. Lipid liking substances are lipophilic while lipid hating substances are termed lipophobic. These properties are instrumental in the formation of the cell membrane.
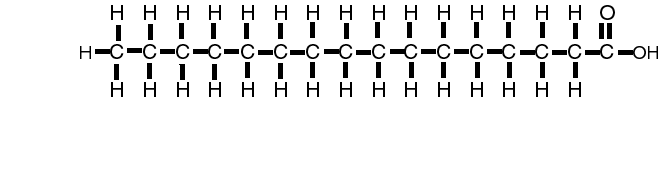
or C16H32O2
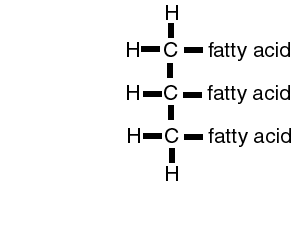
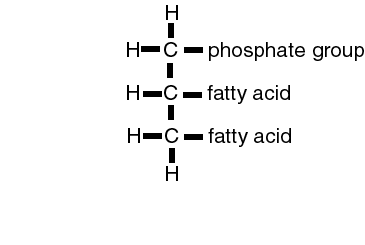
|
Phospholipids
|
Phospholipids are molecules of a fatty acid to which a phosphate group has been attached. Typically, this phosphate group is ionic. An ionic group is hydrophylic, while the fatty acid tail is lipophylic. This give the phospholipid molecule a split personality, part of it is hydrophobic, and part of it is lipophobic. (Which is which?)
|
Cholesterol
|
Cholesterol is a type of fat molecule. It is an integral part of our cell membranes and a precursor to the formation of a variety of hormones, called steroids. Unfortunately, if too much accumulates in the blood stream, it tends to form deposites on the walls of the arteries, a condition known as atherosclerosis.
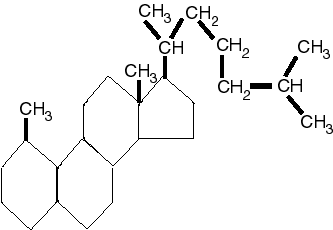
[It is not necessary to memorize the actual molecular structure of these lipids.]
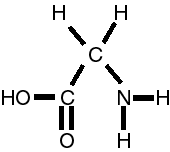
or C2O2H5N or glycine
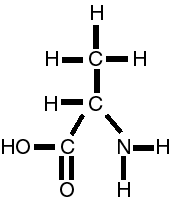
or C3O2H7N or alanine
|
Amino acids I
|
Amino Acids II
|
[It is not necessary to memorize the actual molecular structure of these amino acids.]
|
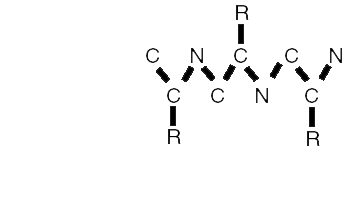
AA Linkages
|
|
Poly Peptide
|
A polypeptide is a chain of amino acids. This string typically "folds up" due to forces between its components amino acids.
|
Peptide Folding
|
Hydrogen Bonds
|
Disulfide Bonds
|
|
Myoglobin
|
|
Ribbon Diagram
|
|
Nucleotides
|
The nucleotide is comprised of a phosphate group, a sugar, and a base. If the sugar is ribose, the molecule is ribose nucleic acid, or RNA. If the oxygen is removed from the sugar ribose, it is called deoxyribose, and the molecule is called deoxyribose nucleic acid, or DNA.
|
Nucleic Acids
|