How Does Adding Impurities Affect Conductivity of Semiconductors?
Now let's consider adding impurities to a semiconductor.
When
we add impurities to semiconductors we call them dopants and the
process is called doping. The result is a dilute (100 -1000 ppm) substitutional solid solution.
There are two kinds of dopants:
one will give negative charge carriers (to make an n-type
semiconductor) and the other will give positive charge carriers (to
make a p-type semiconductor).
N-type Semiconductor
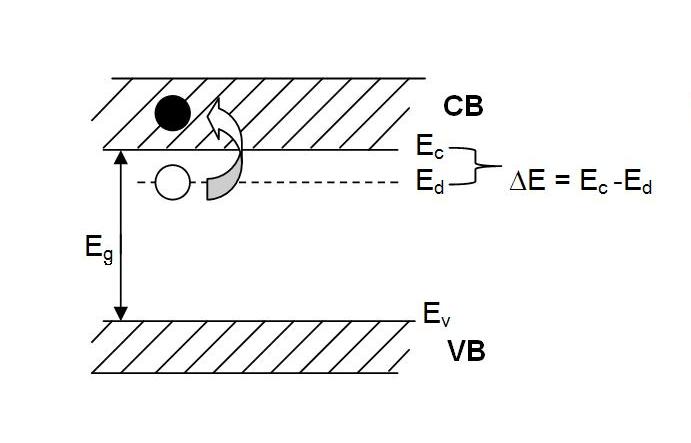
N-type semiconcdutors have dopants from the VA group, such as P+5. These donor impurity atoms are in substitutional solid solution. The extra valance electron not needed for the sp3 tetrahedral bonding is only loosely bound to the P atom in a donor energy level, Ed.
The energy of this donor energy level is close to the lowest energy
level of the conduction band (in Si it is 0.4 eV) and so it is easy to
promote an electron from the donor level to the conduction band. These
promoted electrons become charge carriers that contribute to the
material's conductivity. Since they are negative, the result is
called an n-type semiconductor.
As
temperature increases, more and more of these donor electrons will
be promoted into the conduction band. Eventually, a temperature
will be reached such that there will be none left. The donor electrons
will be "exhausted". During this process the relationship of
conductivity to temperature will look like this:
sigma = sigma0 e – (Ec-Ed) /kT
This is referred to as extrinsic semiconduction. The conductivity depends on the dopants.
After
these electrons from the dopants are all promoted to the conductance band, (i.e. are
exhausted,) there is a range of temperatures before intrinsic
semi-conduction kicks in where the conductivity remains essentially
constant. After that, as temperature increases, there will be a
promotion of electrons from the valance band into the conduction band (intrinsic
behavior).
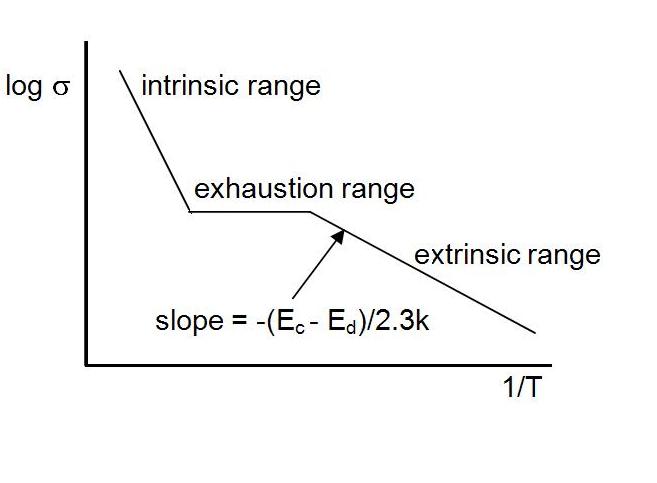
Note
that the temperatures needed to promote the dopant electrons into the
conduction band are lower than the temperatures required to promote the
intrinsic electrons into the conduction band.
Also
note that the slope of the exrinsic range is less steep than the
intrinsic range. This reflects the fact that the activation energy to
promote a dopant electron into the conduction band is less than the
activation energy to promote an intrinsic electron into the conduction
band.
P-type Semiconductor
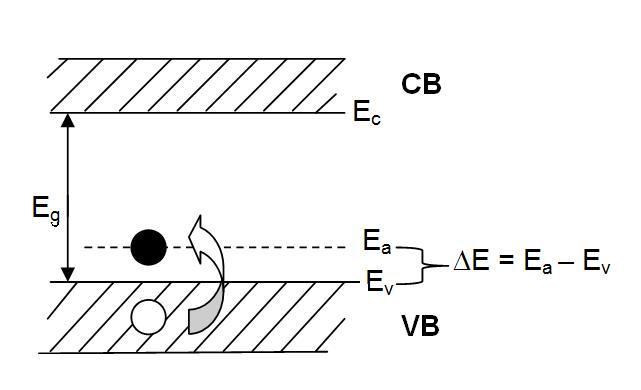
P-type semiconductos have dopants from the IIIA group such as B+3 . These donor impurity atoms in substitutional solid solution. The lack of an electron needed for sp3 tetrahedral bonding is easily filled by a neighboring Si atom into an acceptor energy level, Ea
of the dopant atom.
The energy of this acceptor level is only slightly above the
valance
band and so it is easy to promote an electron from the valance band
into it. For each promotion of an electron into one of these acceptor
levels, a hole is left in the valance band. It is these holes that
become the charge carriers and contribute to the conductivity of the
semiconductor. Since these holes are positive, the result is called a p-type semiconductor.
Note
that the temperatures needed to promote the dopant electrons into the
conduction band are lower than the temperatures required to promote the
intrinsic electrons into the conduction band.
Also
note that the slope of the exrinsic range is less steep than the
intrinsic range. This reflects the fact that the activation energy to
promote a dopant electron into the conduction band is less than the
activation energy to promote an intrinsic electron into the conduction
band.
As
temperature increases, more
and more of electrons from the valance band will be promoted
into these acceptor energy levels. Eventually, a temperature will
be reached such that all the acceptor energy levels will have electrons in
them. The donor acceptor levels will be "saturated". During
this process
the relationship of conductivity to temperature will look like this:
sigma = sigma0 e – (Ea-Ev) /kT
This is referred to as extrinsic semiconduction. The conductivity depends on the dopants.
After the acceptor energy levels have been saturated, there is a range
of temperatures before intrinsic semi-conduction kicks in where the
conductivity remains essentially constant. After that, as
temperature increases, there will be a promotion of electrons from the
valance band into the conduction band (intrinsic behavior).
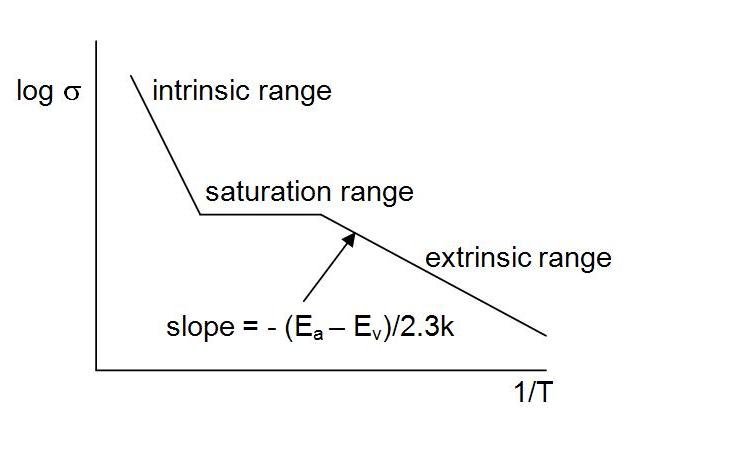
Note
that the temperatures
needed to promote electrons from the valance band into the acceptor
levels (leaving holes in the valance band) are lower than the temperatures required to promote the
intrinsic electrons
into the conduction band.
Also
note that the slope of the exrinsic range is less steep than the
intrinsic range. This reflects the fact that the activation energy to
promote an electron from the valance band into the acceptor level less than the
activation energy to promote an intrinsic electron into the conduction
band.